water drop test organic chemistry|LABORATORY 5 Extraction : wholesalers Liquid-liquid extraction (we will refer to it simply as extraction from now on) is typically conducted with one aqueous phase (either pure water, or . webEurojackpot Results. Eurojackpot draws take place on Tuesdays and Fridays. This page provides details of the results, prize values and number of winners for the last ten .
{plog:ftitle_list}
WEB27 de set. de 2006 · O que é careta: Pessoa antiga, fora da moda, antiquada. Aquele que sempre segue os padrões antigos, que não arrisca coisas novas e diferentes. Definição; Sinônimos; . Aquele menino está fazendo careta para mim 15. 4 9. Careta Significado de Careta Por João Manoel (SP) em 22-11-2009. Compartilhe no Facebook! Compartilhe .
If unsure which layer is aqueous and which layer is organic, do one of the following things: Add a bit of water from a squirt bottle to the separatory funnel (Figure 4.9a) and watch where the water droplets go. If the top layer is . Liquid-liquid extraction (we will refer to it simply as extraction from now on) is typically conducted with one aqueous phase (either pure water, or .In this section are stepwise instructions on how to extract an aqueous solution with an organic solvent that is denser than water (the organic layer will be on the bottom). The following equipment will be utilized by all the senior Bio‐Med, Chemistry and Gateway Senior Organic Chemistry students. The equipment has a number that corresponds .
Tests for Unsaturation
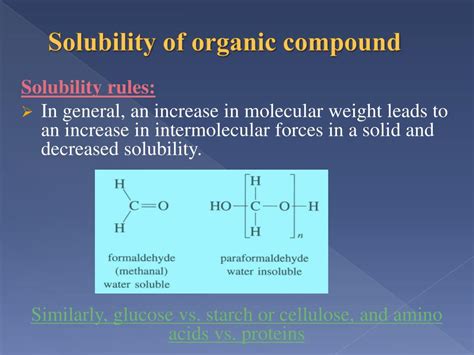
Solubility of Organic Compounds
It is often necessary to separate an organic compound from a mixture of compounds. These mixtures are often derived from natural sources or are the result of synthetic reactions. .This exercise in qualitative analysis will allow you to apply the principles and techniques that you have learned throughout this year in organic chemistry lab. You will not be able to rely on .Water drop test - Used to ascertain which liquid in the separatory funnel is the organic and which is aqueous - Process: add a drop of water through the top of the separatory funnel → if the .
The objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a variety of common organic functional groups. You will then explore the .
minimum volume of water calculated above. Add a boiling stick and begin gently heating on the sand bath. As the solvent begins to boil (see above), add water dropwise until the sample just . How Are Functional-Group Classifications Useful? Classifications by Reaction Types; Contributors and Attributions; There are a number of recurring types of structural features in organic compounds that commonly are known as functional groups.In fact, a traditional approach to the subject of organic chemistry involves the classification of compounds .Chemical Structure of Water. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical formula H 2 O. The arrangement of atoms in a water molecule, shown in Figure \(\PageIndex{2}\), .
bend thumb then wrist compression test
Orgo Lab Quiz 4: Liquid

benefits of compression test
It is commonly used in organic chemistry for several heating purposes, such as evaporating solutions to dryness post-synthesis using a rotary evaporator and removing concentrated samples. Kjeldahl Flask. A Kjeldahl flask is a . liquid in a test tube, incline the test tube and point its mouth away from yourself and your . rest on tabletops. Add acid to water dropwise with constant stirring. 11. If any . Organic Chemistry Laboratory is the most exciting part of the curriculum. It is the place . Test for alkenes. Alkenes will react with bromine water to form a halogenoalkane. Bromine water is orange but the halogenoalkane formed will be colourless. Test for carboxylic acids. Carboxylic acids will react with metal carbonates to produce a salt, water and carbon dioxide. Sodium carbonate is as good a choice as any. In this experiment, we will practice determining which layer is aqueous and which layer is organic.0:23 water + n-butyl chloride1:13 water + n-butyl bromide1.
1. Summary of Alkyne Reactions: Addition, Deprotonation (+ SN2), And Oxidative Cleavage. Like alkenes, the main pathway found in the reactions of alkynes is “addition” – that is, breaking the C-C π bond and forming two new single bonds to carbon. The product of an addition reaction to an alkyne is an alkene – and, as we just mentioned, alkene reactions undergo .
A hose should connect from the water spigot to the lower arm of the condenser, forcing water to travel against gravity through the condenser (this is shown correctly in Figure 5.17b). The hose connecting the upper arm of the condenser should then drain to the sink. By forcing the water uphill, it will completely fill the condenser.Bromine water, also called bromide bromate solution or bromine solution, has the chemical formula Br 2.The molecular weight of bromine water is 159.81, and the density is 1.307 g/mL. Bromine water is a yellow mixture solution with high oxidizing properties, prepared by dissolving diatomic bromine (Br 2) in water (H 2 O).. Bromine water solution can be prepared in the .heating on the sand bath. As the solvent begins to boil (see above), add water dropwise until the sample just dissolves. Add 1 more drop of water. Record the total volume of water needed to dissolve the sample (21 drops = 1 mL) Remove the solution from the heat and place in the test tube holder to cool to room temperature undisturbed.
Figure 6.14: Organic chemistry students determine the melting point of samples. Thiele Tube Method Figure 6.15: a) Capillary sample attached to a thermometer with a small rubber band, b) Placement of the sample in the Thiele tube, c) Correct location of the sample, with an arrow indicating the minimal height of mineral oil in the tube, d .The iodine-potassium iodide solution is prepared from 10 g of iodine and 20 g of potassium iodide in 100 mL of water. Hinsburg Test for . Identification of Organic Compounds", Vogel, "Elementary Practical Organic Chemistry, Part . to dissolve the unknown and add an equal amount of water. Add a drop or two of an appropriate indicator .Organic Chemistry Lab Techniques (Nichols) 6: Miscellaneous Techniques 6.4: Chemical Tests 6.4D: Individual Tests Expand/collapse global location . Note: use water to rinse out the test tubes,and if a red result won't easily clean up, add a few drops of \(6 \: \text{M} \: \ce{HCl}\).
Organic Qualitative Analysis
Temporary Hard Water. Temporary hard water is hard water that consists primarily of calcium (Ca 2 +) and bicarbonate (HCO 3-) ions.Heating causes the bicarbonate ion in temporary hard water to decompose into carbonate ion (CO 3 2-), carbon dioxide (CO 2), and water (H 2 O). The resultant carbonate ion (CO 3 2-) can then react with other ions in the .
Operation Water Drop looks at the chemical contaminants that are found in water; it is designed for a science class. Operation Water Flow looks at how water is used, where it comes from and how much it costs; it has lessons .
Heat the sample in a water bath for a few minutes. Test again for complete precipitation by adding 1 more drop of sulfuric acid. Centrifuge and decant the supernatant. If any further tests will be carried out on the .
Testing for an Aldehyde. Fehling’s solution. Fehling’s solution is an alkaline solution containing copper(II) ions which act as the oxidising agent; When warmed with an aldehyde, the aldehyde is oxidised to a carboxylic acid and . If, however, layers must be discarded, run this simple verification test first. Remove a drop of either layer using a glass pipette (dropper). Add the drop to a small beaker containing distilled water. If the drop dissolves and mixes, you have the aqueous layer; if it forms an observable droplet that does not dissolve, you have the organic layer. Chemistry in Pictures: Drop test . a reaction involving a catalyst that generates hydrogen gas from water runs slowly without gravity driving the produced gas bubbles up and away from the .
Note 1 For our purposes we’ll only discuss normal electron demand Diels Alder reactions, with an electron-rich diene and an electron-poor dienophile. However, there are examples of Diels-Alder reactions that proceed with electron-poor dienes and electron-rich dienophiles. These are known as inverse-electron demand Diels Alder reactions.. Note 2– .Structure of Water. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent (polar bonds).The oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms.
These early examples performed with water as the sole medium differ insofar as several reaction parameters are concerned: (1) Sharpless reported a [2σ + 2σ + 2π] cycloaddition under heterogeneous conditions. 8 (2) Breslow reported a Diels–Alder cycloaddition using homogeneous conditions. 5 (3) Breslow also reported a different behavior depending on the .
benefits of compressive tests vs tensile tests
(2) Drop Formation Method. A drop of liquid is allowed to form at the lower end of a capillary tube (Fig. 2). The drop is supported by the upward force of surface tension acting at the outer circumference of the tube. The weight of the drop (mg) pulls it downward. When the two forces are balanced, the drop breaks. Thus at the point of breaking, For highly accurate and comprehensive results, we recommend the Tap Score Advanced Home Water Test Kit. Water samples are submitted to an EPA-certified laboratory that screens for 114 parameters, providing a much more detailed analysis than at-home test kits. If you have a well, the Health Metric Well Water Test Kit is a great choice. This kit .
Identifying Ketones. Test: Tollen’s solution, Fehling’s solution and acidified potassium chromate (VI) solution. Result: No reaction occurs with Tollen’s solution, Fehling’s solution or potassium chromate (VI) solution Explanation: Ketones are not able to reduce the Ag+ ions, the Cu2+ ions or the Cr6+ ions. Identifying Carboxylic Acids. Test 1 – Limewater: Add a carbonate and bubble .
There are five key factors that influence acidity in organic chemistry; the charge, the atom, resonance, inductive effects, and the orbitals. . HO – is the conjugate base of water. . How Helena Aced Organic Chemistry; From a "Drop" To B+ in Org 2 – How A Hard Working Student Turned It Around; Scientists of the 18th and early 19th centuries studied compounds obtained from plants and animals and labeled them organic because they were isolated from “organized” (living) systems. Compounds isolated from nonliving systems, such as rocks and ores, the atmosphere, and the oceans, were labeled inorganic.For many years, scientists thought organic .
Assistir Ao Vivo Direto Online Sport TV 1 em Portugal - Canal de Desporto disponível apenas em Portugal - Pode aceder ao canal diretamente a partir do site oficial com muitos eventos e notícias exclusivas. ,em alta qualidade - Canal ID: 63105 - celular e desktop grátis, Selecionado por CoolStreaming.us.
water drop test organic chemistry|LABORATORY 5 Extraction